Switching from one generic levothyroxine brand to another might seem like a simple pharmacy swap-same dose, same pill, same price. But for millions of people taking thyroid hormone replacement, that switch can trigger subtle, unsettling changes: fatigue that won’t lift, heart palpitations, unexplained weight gain or loss. The question isn’t whether generics work-it’s whether switching between them requires you to get your TSH tested again.
Why Levothyroxine Is Different
Levothyroxine isn’t like taking an ibuprofen or a blood pressure pill. It’s a narrow therapeutic index (NTI) drug, meaning even tiny changes in blood levels can cause big effects. Too little, and you feel sluggish, cold, and foggy. Too much, and your heart races, you lose weight, and your bones weaken over time. The goal isn’t just to be in the normal range-it’s to be in the right range for you.The standard target for TSH (thyroid-stimulating hormone) is 0.4 to 4.0 mIU/L for most adults. But that’s not universal. Older adults often do better with a higher TSH-up to 6.0 mIU/L. People with thyroid cancer need much tighter control, often under 1.0 mIU/L. Pregnant women need even stricter targets. That’s why a one-size-fits-all approach doesn’t work.
Levothyroxine sodium has the chemical formula C15H11I4NNaO4. It’s a precise molecule, but the tablets you swallow aren’t just pure hormone. They contain fillers, dyes, and binders-excipients-that vary between manufacturers. Mylan, Teva, Pfizer, and Sandoz all make FDA-approved generics. They meet the same bioequivalence rules: their absorption rates must fall within 80-125% of the brand-name version. But for a drug where 12.5 mcg changes how you feel, that 45% window feels wide.
The FDA Says: No Need to Monitor
The FDA has been clear: approved generic levothyroxine products are interchangeable. Their position is based on large studies, including one published in JAMA Internal Medicine in February 2022. That study followed over 15,000 patients who switched between different generic brands. The results? No meaningful difference in TSH levels. The average TSH stayed at 2.7 mIU/L-same for switchers and non-switchers. The percentage of patients with normal TSH? Nearly identical: 64.3% vs. 63.8%.Dr. David S. Cooper, lead author of that study and a thyroid expert at Johns Hopkins, summed it up: “Switching among different generic levothyroxine products was not associated with clinically significant changes in TSH level.” The FDA Commissioner, Robert M. Califf, echoed this in 2023, saying the current bioequivalence standards are sufficient.
In January 2024, the FDA updated levothyroxine labeling to reflect this: “For most patients, switching between different levothyroxine products does not require additional TSH monitoring beyond routine follow-up.” That’s a big shift. It means the default answer for most people is: keep your regular checkup schedule. No extra test needed.
But Some Guidelines Still Say: Test After Switching
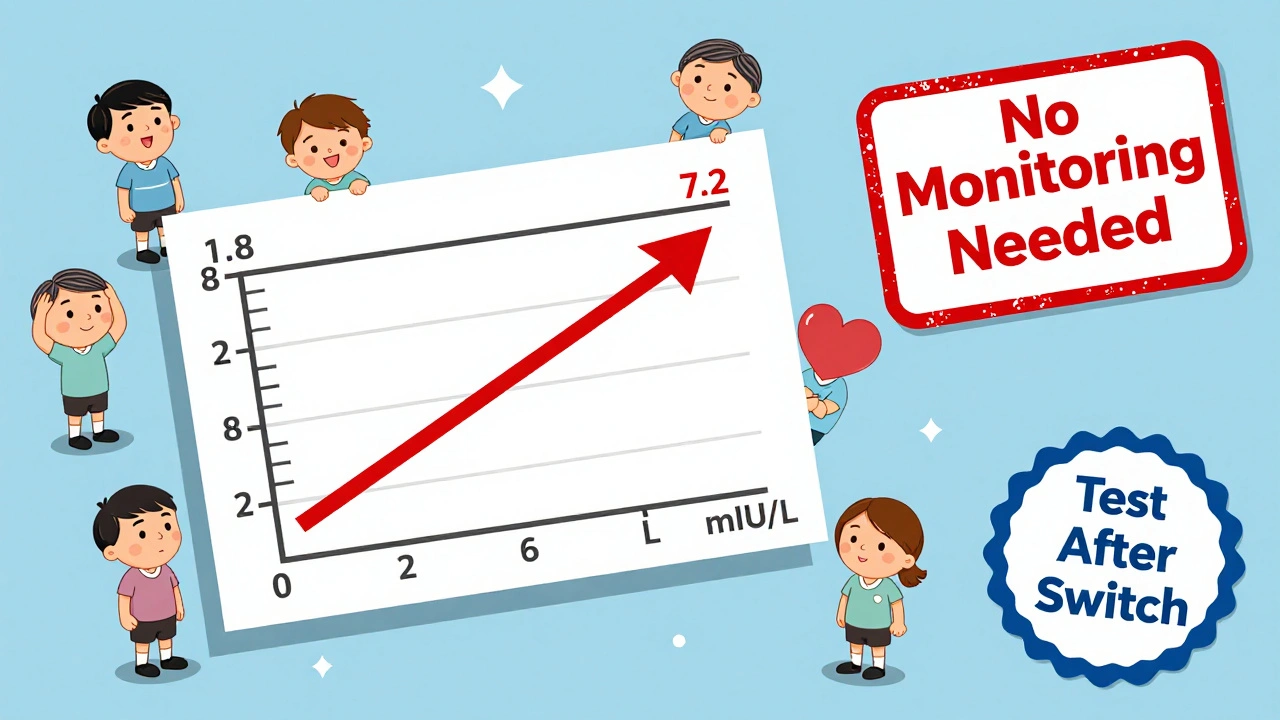
Not everyone agrees. The American Association of Clinical Endocrinologists (AACE) and the American Thyroid Association (ATA) used to recommend checking TSH six weeks after any switch. Their 2014 guidelines warned that switching could alter TSH levels in some patients. Even today, the European Thyroid Association and the European Medicines Agency still advise monitoring 6-8 weeks after a switch.Why the difference? It comes down to caution. Some doctors remember patients who had big TSH spikes after switching-from 1.8 to 7.2 mIU/L, as one Reddit user reported. Others recall patients who developed palpitations or unexplained weight gain. The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) documented over 1,200 suspected adverse reactions linked to product switches between 2015 and 2021. Fatigue, heart issues, and weight changes were the most common.
Paloma Health’s 2021 survey of 1,500 patients found that 18.7% noticed symptoms after switching. About 6.2% needed a dose adjustment. That’s not a majority-but it’s not negligible either. If you’re one of those people, it matters.
Who Should Get a TSH Test After Switching?
You don’t need a test after every switch. But you should if you fall into one of these groups:- Thyroid cancer survivors-TSH targets are strict, often below 0.1 or 0.5 mIU/L. Even a small change can affect outcomes.
- Pregnant women-Thyroid needs rise during pregnancy. Stability is critical for fetal brain development.
- People with severe heart disease-Too much thyroid hormone can trigger arrhythmias.
- Those with a history of TSH instability-If your levels have bounced around before, you’re more likely to react to a new formulation.
- Anyone who feels different after switching-Fatigue, anxiety, weight shift, hair loss, or heart racing? Don’t wait. Get tested.
The American College of Endocrinology’s 2023 guidelines say: monitor TSH 6-8 weeks after a switch for these high-risk groups. For everyone else? Routine annual or biannual checks are enough.
Real Stories: The 8-12% Who React
In online forums like r/Hashimotos, patient stories are everywhere. Some say switching made no difference. Others swear their symptoms vanished only after switching back to a specific brand.One user, ThyroidWarrior89, wrote: “Switched from Mylan to Teva and my TSH jumped from 1.8 to 7.2 in 8 weeks. I had to increase my dose by 12.5 mcg.” Another, HypoNoMore, said: “Switched between three generics in two years. No TSH changes. My doctor says I’m in the 70% who don’t react.”
Research suggests about 8-12% of people are sensitive to formulation changes. Why? It might be:
- Genetic differences in the DIO2 gene, which affects how your body converts T4 to active T3.
- Excipient intolerance-some people react to dyes or fillers, even if they’re FDA-approved.
- Very low thyroid reserve-people whose bodies barely make any hormone on their own.
These aren’t rare. One study found 3.2% of hypothyroid patients have very low reserve. Another found 1.7% report intolerance to excipients. Genetic variants affecting hormone conversion affect less than 1%-but that’s still thousands of people.
What Should You Do?
Here’s a simple, practical plan:- If you’re stable-TSH in range, no symptoms-keep your regular schedule. No extra test needed after switching generics.
- If you’ve just switched and feel off-fatigued, anxious, shaky, gaining weight-get your TSH checked in 4-6 weeks. Don’t wait.
- If you’re in a high-risk group (cancer, pregnancy, heart disease), always get tested 6-8 weeks after any switch.
- If you’ve switched twice and felt fine both times, you’re likely in the majority. Keep going.
- If you’ve switched three times and always had issues? Talk to your doctor about sticking to one brand-even if it costs more.
Pharmacies and insurers push generics because they save money. In 2023, 89% of levothyroxine prescriptions were generic, saving the U.S. system over $2 billion a year. That’s huge. But cost savings shouldn’t come at the cost of patient well-being.
The Bottom Line
Most people can switch between generic levothyroxine brands without a problem. The science supports it. The FDA says so. Large studies confirm it.But not everyone. If you’ve ever felt worse after a switch, or if you’re in a high-risk group, your experience matters more than any statistic. Don’t dismiss your symptoms. Don’t assume it’s “all in your head.” Get tested. Advocate for yourself.
The goal isn’t just to have a normal TSH. It’s to feel normal. If switching brands makes you feel off, that’s not normal. And that’s when you need to act.




9 Comments
Switched from Mylan to Teva last year and my TSH went from 2.1 to 6.9. I felt like a zombie. My doctor said it was ‘just stress’ but I knew better. Got tested, changed back, and now I’m human again. Don’t let anyone tell you it’s all in your head. Your body knows.
Also, why do pharmacies switch meds without telling you? I didn’t even know until I saw the pill color change. 😡
THE FDA IS A PHARMA TOY. They say it’s ‘safe’ because they’re paid off. Look at the history - thalidomide, Vioxx, opioids. They don’t care about you. They care about profit. The 80-125% bioequivalence window? That’s a joke. One pill could be 45% weaker than another. That’s not a generic - that’s Russian roulette with your thyroid.
And don’t get me started on the ‘studies.’ All funded by Big Pharma. Wake up, sheeple.
OMG YES I’M ONE OF THOSE 12%!! Switched to a new generic and I was so tired I napped at my desk. Then I got my TSH checked and it was 8.5 😭 switched back to the old one and boom - energy back. My doc was like ‘hmm maybe?’ and I was like ‘NOPE I KNOW MY BODY’
Don’t wait. Test. You’re worth it. 💪❤️
People in this country are too lazy to advocate for themselves. In Germany, they track every pill. In Japan, they don’t switch without consent. But here? You get whatever’s cheapest. And then you wonder why you’re exhausted. It’s not the medicine - it’s the system.
Stop blaming the FDA. Blame the politicians who let this happen.
Thank you for this detailed and thoughtful post. In India, we often face similar challenges with generic medications. While cost is critical, patient outcomes must not be compromised. For those with thyroid conditions, consistency in formulation is essential. A simple TSH test after switching is a small price to pay for stability and well-being.
Always prioritize health over savings when dealing with narrow therapeutic index drugs.
The real issue isn’t whether generics work - it’s that we treat thyroid patients like data points. The FDA’s stance is statistically valid, but medicine isn’t statistics. It’s biology. It’s lived experience. A 12.5 mcg difference might not move the average TSH, but for someone with zero thyroid reserve, that’s everything.
We need a system that honors individual biology, not just population averages. The 8-12% who react aren’t outliers - they’re the canaries in the coal mine.
Just switched and felt weird - got tested. TSH was 5.8. Changed back to my old brand. Back to normal in 2 weeks. You don’t need a PhD to know your body. Trust yourself. 💕
hmm… interesting. i mean, the data says one thing, but my friend’s aunt had a meltdown after switching - like, full-on panic attacks and hair falling out. so… maybe the stats are right, but the human part? that’s where it gets messy. i dunno. just saying.
also, typo: ‘excipents’ - should be ‘excipients’ 😅
As a board-certified endocrinologist with over 20 years of clinical experience, I must emphasize: the FDA’s position is not only scientifically sound, but it is also the only rational policy given the cost burden on the healthcare system. Patients who report symptoms after switching are often suffering from psychosomatic manifestations or non-adherence.
Moreover, the notion that excipients cause clinical effects is a myth perpetuated by internet forums. The FDA’s bioequivalence criteria are rigorous and validated across thousands of patients.
Do not confuse anecdotal reports with evidence-based medicine. Your thyroid is not a magic potion - it’s a hormone. Treat it like one.