CGMP: What It Means for Generic Drug Safety and Quality
When you pick up a generic pill, you expect it to work just like the brand-name version. That’s not luck—it’s because of CGMP, Current Good Manufacturing Practices, the set of rules that control how pharmaceuticals are made. Also known as cGMP, it’s the backbone of every approved generic drug in the U.S. and beyond. Without CGMP, there’s no way to guarantee that the medication you’re taking isn’t contaminated, under-dosed, or made in unsanitary conditions.
CGMP isn’t just paperwork—it’s a live system that covers every step: from the raw ingredients coming in, to how machines are cleaned between batches, to how workers are trained and how records are kept. The FDA, the U.S. agency that enforces drug safety standards doesn’t just approve drugs once and walk away. They inspect factories without warning, review lab data, and track complaints from patients and doctors. If a batch fails CGMP standards, the FDA can pull it off shelves fast. That’s why posts like How the FDA Monitors Generic Drug Safety After Approval and When Your Doctor Might Prescribe Brand-Name Only matter—they show how CGMP gaps can lead to real-world risks, like inconsistent dosing in generics or dangerous interactions because of hidden ingredients.
CGMP also connects to how drugs are labeled, stored, and even disposed of. If a pill bottle has your name and address printed on it, that’s fine—until someone steals it. That’s why How to Disable Personal Information on Medication Bottles is a practical follow-up to CGMP: if the drug is made right but the packaging leaks your identity, the system still fails. Similarly, when you check the FDA Drug Shortage Database, a public tool to track medication availability, you’re seeing the ripple effect of CGMP failures—like a factory shutdown due to contamination that leaves thousands without their meds. Even something as simple as how a steroid cream is mixed (like in Candid B Lotion vs Alternatives) falls under CGMP. Too much or too little of the active ingredient? That’s a violation.
CGMP isn’t about making drugs expensive—it’s about making them reliable. That’s why you’ll find posts covering everything from Statins and Antifungal Medications (where drug interactions can turn deadly if manufacturing purity isn’t controlled) to How to Shop Pharmacies for the Best Cash Price (where cheap prices can sometimes mean dodgy sourcing). The real question isn’t whether generics are cheaper than brand names—it’s whether they’re made to the same standard. And that’s where CGMP steps in. Below, you’ll find real stories, real data, and real advice on how these rules affect your health, your wallet, and your peace of mind.
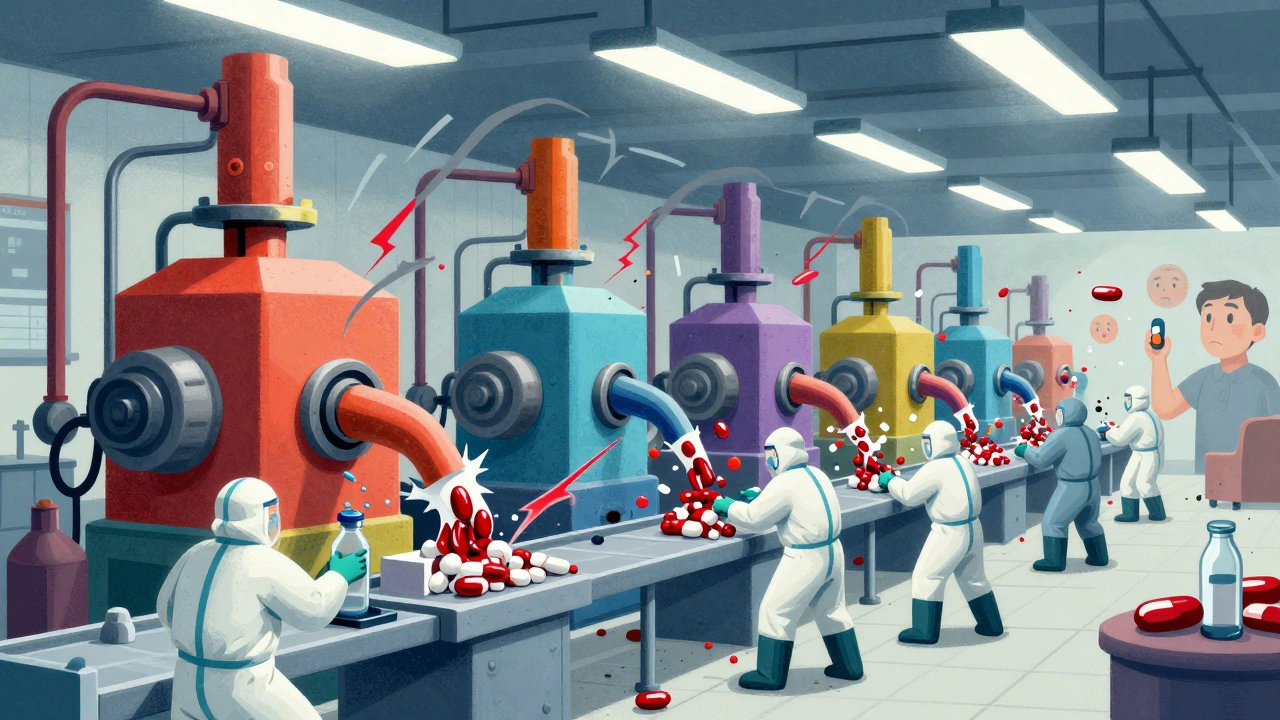
Contamination Controls: Preventing Adulteration in Generic Drug Manufacturing
Contamination controls in generic drug manufacturing prevent harmful adulteration through cleanroom standards, real-time monitoring, and strict cleaning protocols. Learn how facilities avoid cross-contamination and meet FDA requirements.
read more