
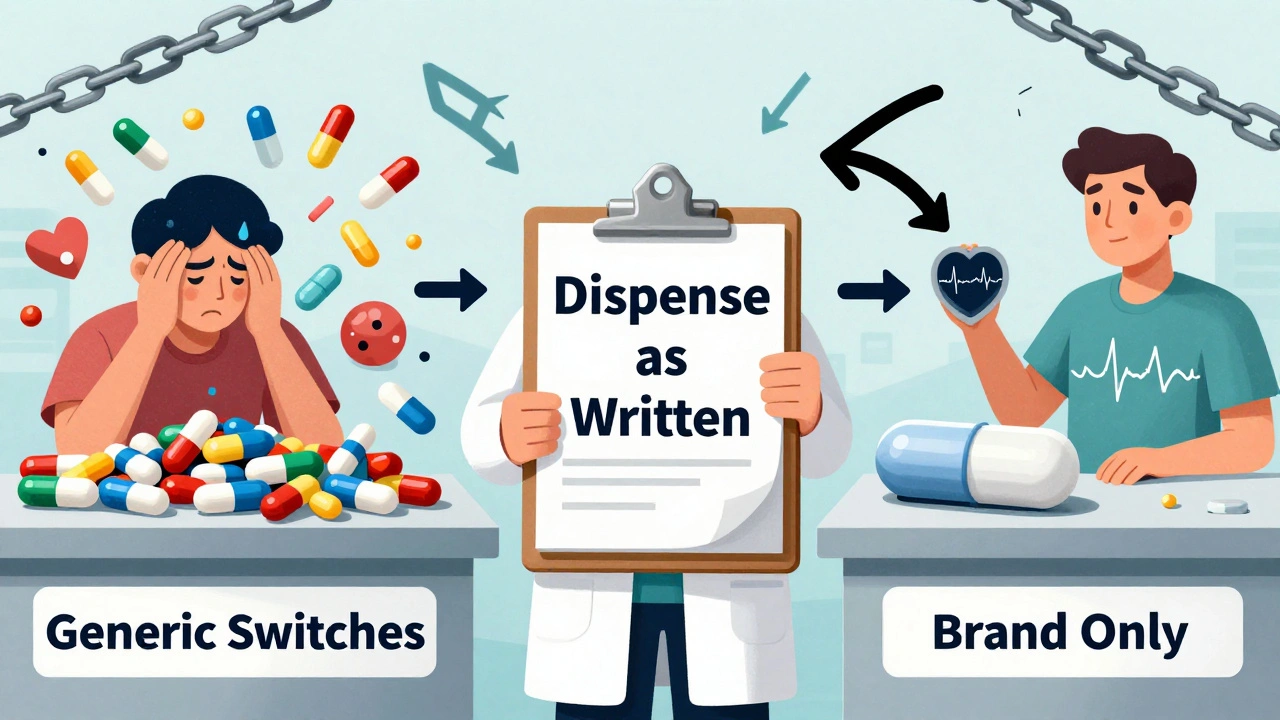
Most prescriptions you get filled are generics. They’re cheaper, just as effective, and approved by the FDA. But sometimes, your doctor writes a prescription that says brand-name only. No substitutions. Why? It’s not because they’re being old-fashioned or influenced by drug reps-at least not always. There are real, science-backed reasons why some medications need to stay as the original brand, even when a generic version exists.
When Generic Substitution Can Be Risky
Not all drugs are created equal, even when they contain the same active ingredient. For some medications, tiny differences in how the body absorbs them can mean the difference between working perfectly and failing dangerously. These are called narrow therapeutic index (NTI) drugs. A small change in blood levels can lead to side effects, loss of control, or even life-threatening complications. Examples include:- Levothyroxine (Synthroid): Used for thyroid conditions. Even small changes in absorption can cause fatigue, weight gain, or heart problems.
- Warfarin (Coumadin): A blood thinner. Too much increases bleeding risk; too little raises the chance of clots.
- Levetiracetam (Keppra): An anti-seizure medication. Switching to a generic triggered breakthrough seizures in nearly 13% of patients in one study, compared to just 4% who stayed on the brand.
The FDA says generics must be within 80-125% of the brand’s absorption rate. That sounds fine-until you’re talking about a drug where a 10% shift can make you sick. The American Thyroid Association and the American Academy of Neurology both recommend sticking with the same brand for these drugs. Not because generics are bad-but because consistency matters.
Why Doctors Still Say ‘Dispense as Written’
Doctors don’t write ‘do not substitute’ on a whim. It’s usually based on one of three things:- Previous patient reaction: If you’ve tried a generic version before and had side effects-like nausea, dizziness, or mood changes-it’s not a guess. It’s a documented pattern. One patient on Reddit said switching from Synthroid to generic caused severe depression twice. She went back to the brand, and her symptoms vanished.
- Inactive ingredients: Generics use different fillers, dyes, or binders. For most people, this doesn’t matter. But for someone with a sensitivity-say, to lactose or a specific dye-it can trigger stomach issues, rashes, or allergic reactions. This is especially common with antibiotics like ciprofloxacin, where different generic makers use different inactive ingredients.
- Delivery systems: Some drugs aren’t just about the chemical. Think of Advair’s Diskus inhaler or insulin pens. The way the drug is delivered can affect how much actually reaches your lungs or bloodstream. Generics may not replicate the exact device, even if the drug inside is the same.
These aren’t theoretical concerns. A 2022 study in the Annals of Internal Medicine found that patients who switched generics for NTI drugs were 2.5 times more likely to be hospitalized for complications. That’s why doctors who treat epilepsy, thyroid disease, or heart conditions are especially cautious.
The Cost Difference Is Huge-But Not Always Worth It
Let’s talk numbers. In 2022, the average brand-name prescription cost $471.67. The generic? Just $13.76. That’s an 85% drop. For statins, blood pressure meds, or diabetes drugs like metformin, switching saves patients hundreds-sometimes over $1,000 a year.A 2020 JAMA meta-analysis of over 112,000 patients found no difference in outcomes between brand and generic versions of common drugs like lisinopril, atorvastatin, or omeprazole. For these, generics are just as safe and effective.
But here’s the catch: 90% of all prescriptions filled in the U.S. are generics. Yet they make up only 23% of total drug spending. That means brand-name drugs-despite being used far less-still drive most of the cost. And when a doctor prescribes a brand-name drug unnecessarily, it’s not just expensive for you. It’s expensive for the whole system.
Insurance and the Bureaucratic Hurdle
If your doctor writes ‘brand medically necessary,’ your insurance doesn’t just automatically pay for it. They’ll likely require prior authorization. That means your doctor has to fill out paperwork explaining why. The process can take up to 72 hours. Approval rates vary: 89% for anti-seizure drugs, but only 45% for acid reflux meds like omeprazole.Patients often don’t realize this. They leave the office thinking they’ve gotten a special prescription. But if the insurer denies it, they’re stuck paying full price-or switching anyway. A 2021 Kaiser Family Foundation survey found 42% of patients ended up paying more out-of-pocket because their doctor prescribed a brand without checking insurance rules first.
And here’s another twist: some pharmacies won’t even tell you they switched your medication. In 37% of negative reviews on Drugs.com, patients blamed inconsistent side effects on generics-only to find out later that their pharmacist had switched brands multiple times, even though the doctor didn’t authorize it.

What’s Changing? And What’s Not
The FDA is trying to fix some of the confusion. In 2023, they started requiring generic manufacturers to match the shape and color of brand-name pills to reduce patient errors. That’s a big deal-because if your pill looks different, you might think it’s not working, or worse, stop taking it.There’s also a new option: authorized generics. These are made by the original brand company but sold under a generic label. They’re identical to the brand-same inactive ingredients, same manufacturing line. They’re not cheaper than regular generics, but they’re more consistent. Some insurers are starting to cover them as a middle ground.
Still, the problem isn’t just about science. It’s about habits. A 2018 study found doctors use brand names in 15-20% of prescriptions-even when guidelines say to use generics. Why? Because it’s easier. The brand name is what they learned in med school. It’s what’s on the drug rep’s card. It’s what’s in their head.
And patients? They often ask for the brand. They’ve seen the ads. They think ‘name brand’ means better. But for most drugs, that’s just marketing.
What You Can Do
If your doctor prescribes a brand-name drug:- Ask why. ‘Is this because of my history? Or because it’s what you usually prescribe?’
- Check if it’s an NTI drug. If it’s for thyroid, epilepsy, or blood thinning, stick with the brand unless your doctor says otherwise.
- Ask about authorized generics. They’re a good compromise if cost is an issue.
- Don’t assume the pharmacist won’t switch. Always check the pill’s appearance and name on the label. If it’s different from last time, ask.
- Use tools like GoodRx or the FDA’s Orange Book to compare costs and therapeutic ratings before you fill it.
Most of the time, generics are perfect. But for a small group of medications, the difference isn’t just about price-it’s about safety. Knowing when to insist on the brand, and when to save money, is part of being your own health advocate.
Can my pharmacist substitute a generic if my doctor didn’t say ‘do not substitute’?
Yes, in 49 U.S. states and Washington D.C., pharmacists can legally switch to a generic unless the doctor writes ‘dispense as written,’ ‘do not substitute,’ or ‘brand medically necessary.’ Texas has different rules for certain drugs. Always check your prescription label to confirm what was written.
Are generic drugs really as good as brand-name ones?
For most medications-like statins, blood pressure pills, and antibiotics-yes. The FDA requires generics to have the same active ingredient, strength, and absorption rate as the brand. Studies involving over 100,000 patients show no difference in effectiveness or safety. But for narrow therapeutic index drugs like levothyroxine or warfarin, consistency matters more, and switching can carry risks.
Why do some people have bad reactions to generics?
The active ingredient is the same, but the inactive ingredients-like fillers, dyes, or preservatives-can differ between manufacturers. Some people are sensitive to these, leading to stomach upset, rashes, or mood changes. This is especially common with antibiotics and thyroid meds. If you notice side effects after switching, tell your doctor and pharmacist.
What’s an authorized generic?
An authorized generic is made by the original brand-name company but sold without the brand name. It’s identical to the brand in every way-including inactive ingredients. It’s not cheaper than a regular generic, but it eliminates variability between manufacturers. Some insurers cover it as a middle ground between brand and generic.
Why does my doctor keep prescribing the brand if generics are cheaper?
Sometimes, it’s because they’re unaware a generic is available. A 2021 study found doctors correctly identified generics for only 63% of common drugs. Other times, it’s habit-brand names are easier to remember. Or, it could be patient pressure. Many people believe brand names are better, even when they’re not. Always ask if there’s a generic option and why the brand is being chosen.




15 Comments
It’s fascinating how something as seemingly simple as a pill’s filler can trigger whole-body reactions. I’ve seen patients on warfarin go from stable INRs to dangerous spikes after a generic switch-not because the active ingredient changed, but because the new manufacturer used a different binder that altered dissolution kinetics. The FDA’s 80–125% range sounds precise until you realize that for NTI drugs, that window is a canyon. Consistency isn’t luxury-it’s survival.
And honestly, the fact that pharmacies can swap without telling you is terrifying. I once had a patient panic because her thyroid med looked different. Turned out, her pharmacist had switched brands four times in six months. No one logged it. No one warned her. We’re treating pharmacology like grocery shopping.
It’s not that generics are bad. It’s that the system treats them like interchangeable commodities when they’re not. We need better tracking, better communication, and mandatory patient notification when substitutions occur. This isn’t about fearmongering-it’s about harm reduction.
And yes, I know the cost savings are massive. But when someone ends up in the ER because their levetiracetam dose didn’t hold, who pays then?
It’s a system failure, not a drug failure.
The pharmacokinetic variability inherent in generic substitution for narrow therapeutic index (NTI) agents is not merely a clinical nuance-it is a quantifiable risk factor with statistically significant morbidity and mortality implications. The 2022 Annals of Internal Medicine study referenced demonstrates a 2.5-fold increase in hospitalizations following generic switches, which is not an artifact but a reproducible outcome across multiple cohorts.
Moreover, the FDA’s bioequivalence threshold of 80–125% AUC and Cmax is rooted in population-level statistics, not individual pharmacogenomic profiles. For patients with CYP2C9 or CYP2C19 polymorphisms, even minor shifts in absorption kinetics can precipitate catastrophic therapeutic failure or toxicity. Levothyroxine, for instance, exhibits nonlinear absorption kinetics influenced by gastric pH, food timing, and enteric binding agents-all variables that differ across generic formulations.
The American Thyroid Association’s position is not reactionary; it is evidence-based. The same applies to antiepileptics: the 13% breakthrough seizure rate in the Keppra study was not anecdotal-it was prospectively measured in a multicenter trial.
Authorized generics represent the only viable compromise: identical formulation, same manufacturing line, no bioequivalence gamble. Yet insurers resist coverage because they still conflate ‘generic’ with ‘low-cost’ rather than ‘therapeutically equivalent.’
Until regulatory frameworks evolve to recognize NTI drugs as a distinct pharmacologic class, patient safety will remain subordinate to cost containment metrics.
so like... if you take thyroid meds and switch to generic and feel like crap, its not you being dramatic? its the filler? wow. i always thought people were just being lazy or paranoid. turns out i was the dumb one. thanks for the education. i just hope my doc knows this stuff.
also why do pharmacies not tell you they switched? that feels sketchy. like theyre hiding something. i wouldnt trust them with my dog’s medicine if they did that.
Ah, the great American pharmaceutical paradox: we demand innovation and precision in our smartphones, yet we treat life-sustaining medication like bulk coffee beans-swapping brands willy-nilly because the price differential is 0.3 cents per pill.
It’s not just about bioequivalence-it’s about the epistemology of trust. When your pill changes color, shape, or even the faint odor of the coating, your brain interprets it as ‘this isn’t the same.’ And for NTI drugs, that psychological cue is often physiologically valid.
Authorized generics? Brilliant. Why aren’t they the default? Because the system rewards opacity. The brand-name companies profit from the fear of generics. The insurers profit from the illusion of savings. And patients? We’re the ones left wondering why we feel like zombies.
It’s not capitalism. It’s pharmaceutical feudalism.
Also, I’ve seen people switch from Synthroid to generic and then blame their therapist for ‘not helping enough.’ The body remembers. The mind just rationalizes.
PS: I’m not anti-generic. I’m pro-consistency. And pro-transparency. And pro-not-letting pharmacists play Russian roulette with my endocrine system.
As someone from India where generic medicines are the backbone of public health, I find this discussion both familiar and deeply concerning. In our system, generics are not just affordable-they’re essential. But we also have a parallel reality: many patients rely on the same manufacturer’s product for years, even if it’s technically a generic, because pharmacists know their stability and consistency.
What’s missing here is cultural context. In the U.S., patients are told to expect variability. In India, patients are taught to recognize and demand consistency. We don’t have the luxury of brand-name drugs for most, so we develop deep loyalty to specific manufacturers-not because of marketing, but because of survival.
Perhaps the solution isn’t just authorized generics, but a global standard for manufacturer transparency. A QR code on the bottle that tells you the exact source, batch, and inactive ingredients. That would empower patients everywhere.
And yes, for NTI drugs, the difference is real. But so is the dignity of access. We must not let cost become the enemy of safety, nor safety the enemy of equity.
THIS IS WHY I KNOW THE PHARMA COMPANIES ARE LYING TO US!!!
They don’t want you to know that generics are just as good-because then they’d lose their monopoly profits! The FDA is in their pocket! The 80-125% range? That’s a loophole designed to let them poison people with cheap junk! They don’t care if you have seizures or heart attacks-they care about quarterly earnings!
And now they’re pushing ‘authorized generics’ like it’s some miracle? HA! It’s the same company making the same drug under a different label to trick you into thinking you’re saving money while they keep the price high!
Why don’t they just make ONE standard generic that’s FDA-certified for NTI drugs? Because they don’t want to! They want you confused! They want you scared! They want you paying $471 for Synthroid when it’s just T4!
Wake up, people! This isn’t medicine-it’s corporate extortion with a white coat!
And don’t even get me started on the ‘inactive ingredients’ excuse-those are just the toxins they add to make you dependent! Lactose? Dyes? They’re not ‘fillers’-they’re psychological triggers to make you think you need the brand!
STOP TRUSTING THE SYSTEM. START TRUSTING YOURSELF.
And if your doctor prescribes brand? Ask them if they’re paid by the drug rep. I bet they are.
They let Chinese factories make our meds and then act surprised when people get sick? Of course the generics are sketchy. We outsourced everything. Now we’re paying the price. And the FDA? They’re just rubber-stamping it. This is why America’s falling apart. No standards. No pride. Just cheap junk from overseas.
And now they want us to trust some random pill that looks different? No thanks. I’ll pay the extra $10. At least I know it’s made in the USA.
Someone’s gotta protect this country. It ain’t gonna be the government.
This is such a nuanced issue-and honestly, one of the most under-discussed public health topics. The real problem isn’t generics or brands-it’s the lack of pharmacovigilance infrastructure. We track adverse events for new drugs, but not for generic switches.
What if we had a national registry where patients could report changes in symptoms after a formulation switch? Imagine if every time a pharmacist substituted a generic, the patient received a link to a simple survey: ‘How do you feel compared to last month?’
That data could be anonymized and aggregated. We could identify high-risk generics in real time. We could flag manufacturers with inconsistent dissolution profiles. We could empower clinicians with real-world evidence, not just clinical trial data.
And if we made that data public? Patients could choose based on manufacturer reliability-not just price.
It’s not about being anti-generic. It’s about being pro-transparency. And pro-science. And pro-patient agency.
Let’s turn this from a fear-based conversation into a data-driven one.
the thing no one talks about is how hard it is to even notice when your med changes. you take your pill every day. you don’t look at it. you don’t compare. you just swallow it. then one day you feel off. tired. weird. anxious. you think it’s stress. or aging. or your brain. you don’t think it’s the pill. until you see the name on the bottle and realize it’s different.
and then you have to call your doctor. your pharmacist. your insurance. explain it all over again. and they all say ‘oh that’s fine’ even though you know it’s not.
why isn’t there a simple way to know? a notification? a text? a sticker on the bottle? why is it our job to be pharmacists?
it’s not just about science. it’s about dignity.
you should be able to trust your medicine.
It’s strange how we’ve turned medicine into a commodity while treating the human body like a sacred temple. We’ll spend $800 on a mattress because ‘sleep quality matters,’ but balk at $20 extra for a thyroid pill because ‘it’s just a generic.’
Yet the body doesn’t care about your budget. It cares about consistency. About rhythm. About the quiet, invisible ballet of absorption, metabolism, excretion.
Levothyroxine isn’t just a hormone replacement-it’s the conductor of your entire endocrine orchestra. Change the baton, and the symphony goes off-key. No one tells you that when you’re handed a new bottle.
And the color change? That’s not just about recognition. It’s about ritual. The pill is your daily prayer. If it looks different, you wonder: Is this still me?
Maybe the answer isn’t more regulation. Maybe it’s more reverence.
We don’t need more science. We need more care.
in india we have this thing called ‘generic chain pharmacies’ where you get the same manufacturer’s version every time. even if it’s not branded, you know it’s from the same company. no surprises. no panic. just consistency. maybe the us needs something like that. not for cost. for peace of mind. 🙏
also i love how people say ‘just ask your doctor’ like that’s easy. sometimes you’re scared. sometimes you’re tired. sometimes you just want the pill to work without having to be a detective.
we need better systems. not just better patients.
sooo... i have to pay $471 for a pill that costs $13? and the system is okay with that? 😭
also why is everyone acting like this is new info? i’ve been on generic keppra for 5 years and never had a seizure. so maybe it’s just me? or maybe people are just scared of change?
also why does this feel like a rich person’s problem? like, i can’t even afford the generic sometimes 😂
also also: why are you all so serious? it’s a pill. not a nuclear bomb. 😅
You people are ridiculous. You think because you have a doctor’s note, you’re special? Everyone else takes generics and lives fine. You’re just weak. If your body can’t handle a generic, maybe you’re not meant to be healthy. Stop whining. Take the cheap pill. Be grateful. The world doesn’t owe you Synthroid.
And stop blaming the pharmacist. If you can’t read the label, that’s your problem.
Also, authorized generics? That’s just a fancy word for ‘still expensive.’ Why not just take the real generic and stop being a baby?
Grow up.
The FDA’s bioequivalence standards for generics are a national disgrace. The 80–125% range is not science-it’s corporate lobbying masquerading as regulation. When the same company that lobbied against Medicare negotiation is also writing the rules for drug absorption thresholds, you don’t have a regulatory body-you have a cartel.
And yet, the medical establishment continues to treat this as a neutral, evidence-based policy. No. It is a moral failure.
Patients are not lab rats. They are not cost centers. They are human beings who deserve predictable, reliable, consistent treatment.
It is not ‘inconvenient’ to require identical formulations for NTI drugs. It is not ‘expensive’ to require transparency. It is not ‘unrealistic’ to expect informed consent.
This is not a debate about pharmacology.
This is a debate about whether we value human life over corporate profit.
And the answer, clearly, is no.
That last comment? That’s the one that made me cry.
Because it’s not about the pill.
It’s about what we’ve become.
We used to say, ‘First, do no harm.’
Now we say, ‘First, do no cost.’
And we wonder why people don’t trust medicine anymore.